Prescribing Information
This site is intended for US Healthcare Professionals only
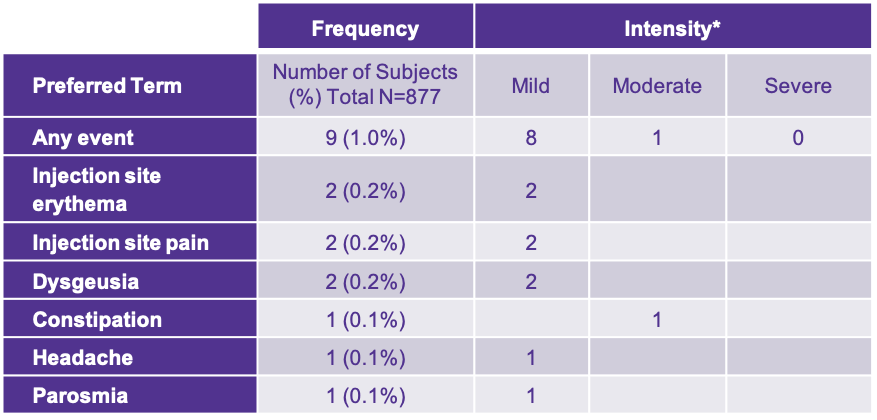
*As assessed by investigator and reported in 18F-fluciclovine NDA.
No overall differences in safety or effectiveness were observed between older adults and younger subjects.1
Adverse reactions were reported in ≤1% of subjects during clinical studies with Axumin. The most common adverse reactions were injection site pain, injection site erythema, and dysgeusia (abnormal sense of taste).1
To report suspected adverse reactions to Axumin, call 1-855-AXUMIN1 (1-855-298-6461) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
A negative image does not rule out the presence of recurrent prostate cancer and a positive image does not confirm the presence of recurrent prostate cancer. The performance of Axumin seems to be affected by PSA levels. Fluciclovine F 18 uptake is not specific for prostate cancer and may occur with other types of cancer and benign prostatic hypertrophy in primary prostate cancer. Clinical correlation, which may include histopathological evaluation of the suspected recurrence site, is recommended.1
Axumin® (fluciclovine F 18) injection is indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment.
To report suspected adverse reactions to Axumin, call 1-855-AXUMIN1 (1-855-298-6461) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Axumin full Prescribing Information.
You are now leaving Axumin.com. Do you wish to proceed?