Prescribing Information
This site is intended for US Healthcare Professionals only
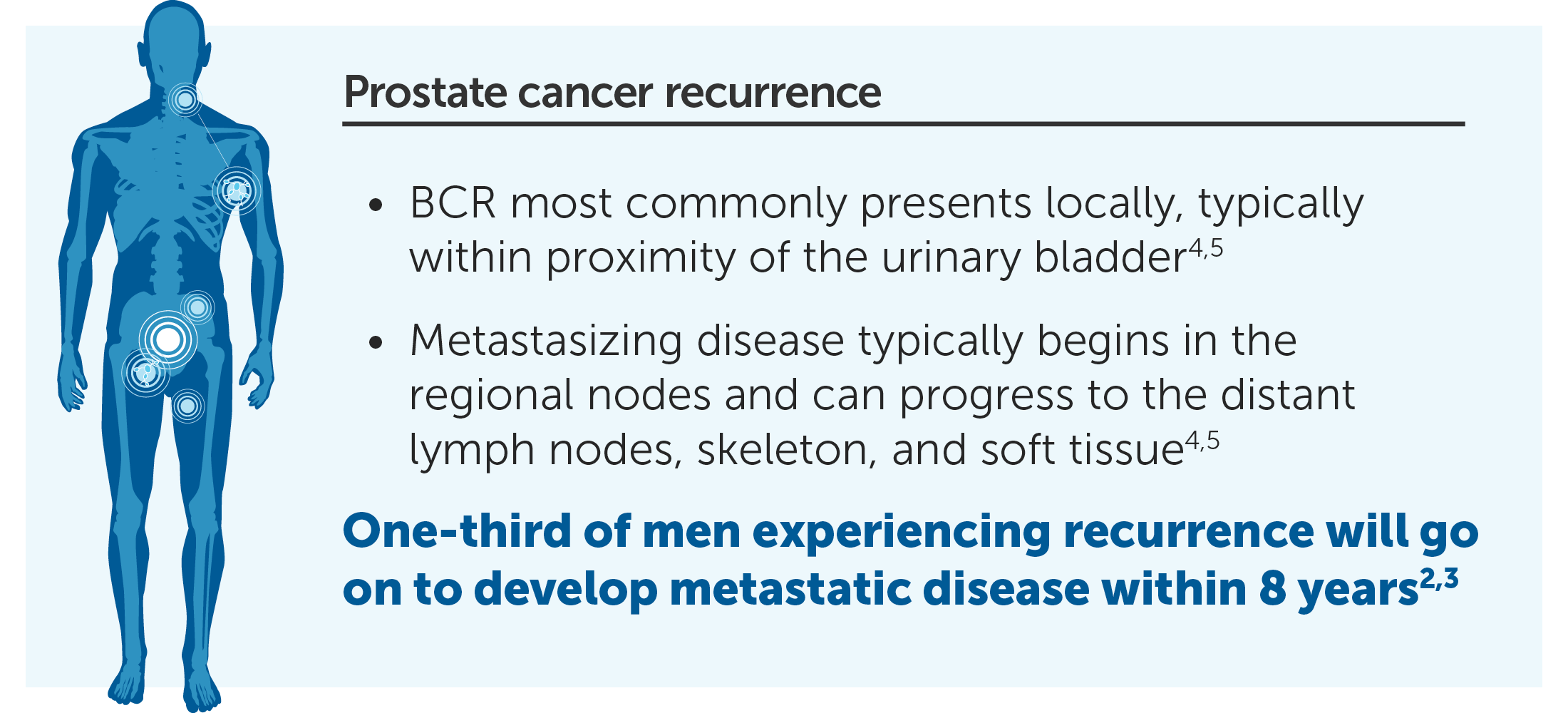
20% to 40% had undergone a radical prostatectomy
30% to 50% had been treated with radiation
Many traditional approaches still present challenges
|
May not detect small tumors |
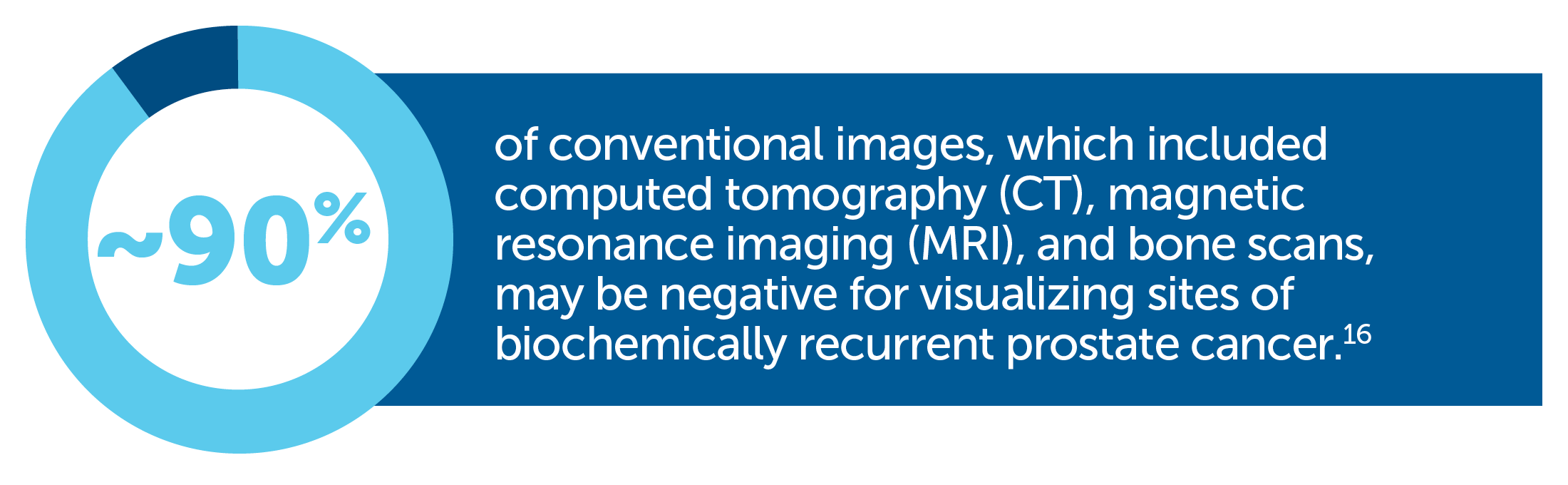
Some imaging procedures may be unable to detect recurrent prostate tumors <1 cm in size or when PSA levels are <10 ng/mL—when cancer may be more effectively managed or treated with localized therapy7-12 |
|
Difficult to use |
Some imaging procedures can be time-consuming or present challenges for reproducibility of results13-15 |
|
May require multiple scans to evaluate all potential metastatic sites |
Bone scans, CT scans, and/or MRI may be necessary7 |

Axumin® (fluciclovine F 18) injection is indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment.
To report suspected adverse reactions to Axumin, call 1-855-AXUMIN1 (1-855-298-6461) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Axumin full Prescribing Information.
You are now leaving Axumin.com. Do you wish to proceed?