Prescribing Information
This site is intended for US Healthcare Professionals only
When presented with a negative or equivocal scan, Axumin provides clarity for decision-making in clinical management.1
Clinical history:
1st PSA recurrence and treatment:
2nd PSA recurrence:
Conventional imaging results:
Planned clinical management:
Details associated with decision to refer patient for an Axumin scan:
Axumin imaging interpretation:
Final management plan:
Clinical follow-up:
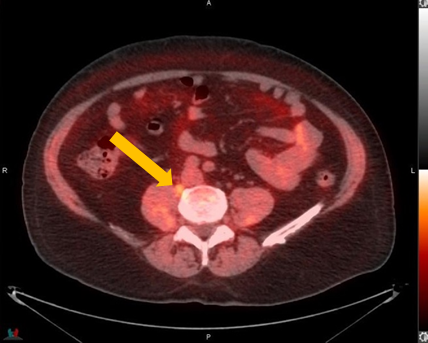
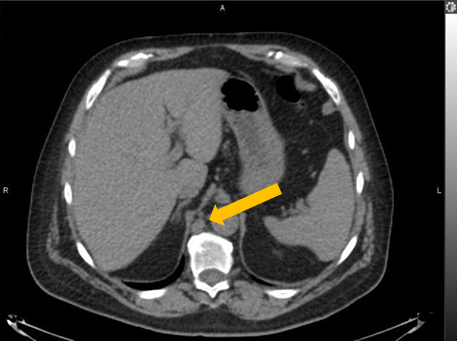
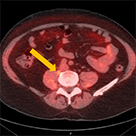
Axumin imaging revealed para-caval lymph node malignant uptake
Lymph Node SUV(bw)max: 2.7
Blood pool SUV(bw)mean: 1.0
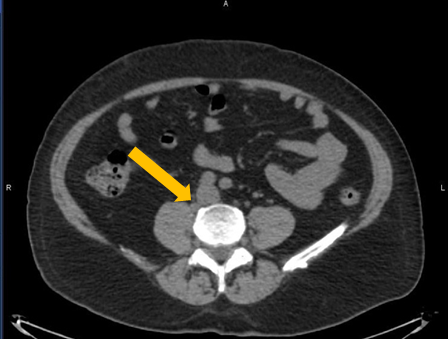
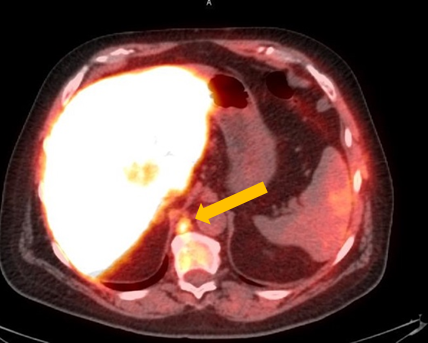
Axumin imaging revealed retrocrural lymph node malignant uptake
Lymph Node SUV(bw)max: 3.6
Blood pool SUV(bw)mean: 1.0
Provided by:
David Josephson, MD, FACS Tower Urology Institute for Minimally Invasive and Robotic Surgery / Attending Surgeon / Cedars Sinai Medical Center / Los Angeles, CA
Jennifer Kujak, MD Director of Oncological Imaging / RadNet Los Angeles / Los Angeles, CA
Clinical history:
PSA at consultation:
Conventional imaging results:
Planned clinical management:
Details associated with decision to refer patients for an Axumin scan:
Axumin imaging interpretation:
Final management plan:
Clinical follow-up:
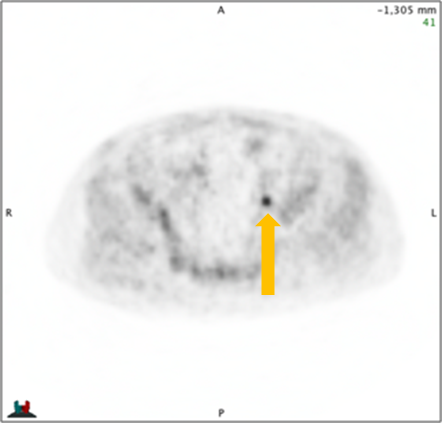
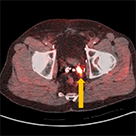
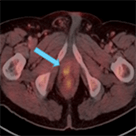
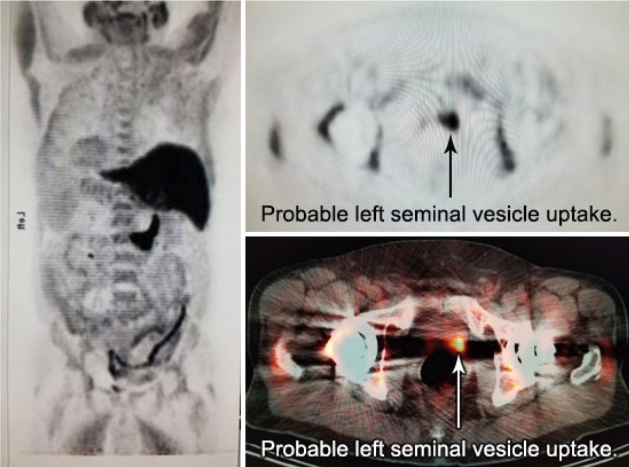
Axumin imaging revealed avid disease within the left seminal vesicle (3/2017)
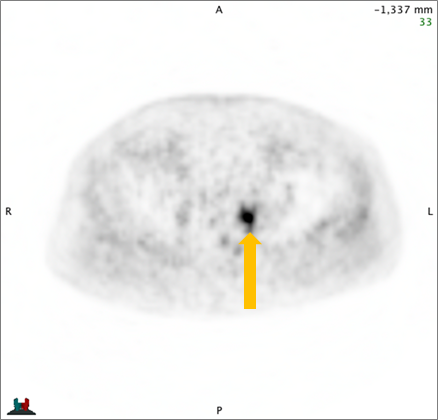
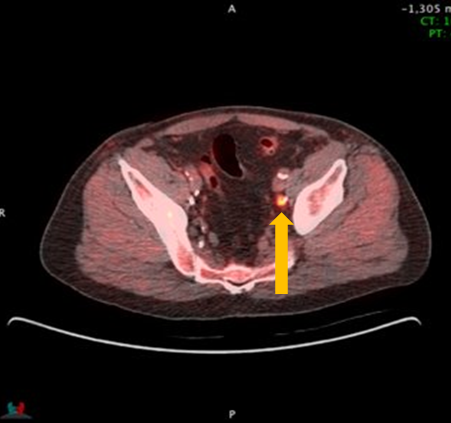
Axumin imaging revealed avid 8mm lymph node along the superior left pelvic side wall, adjacent to a surgical clip (3/2017)
Provided by:
Barry A. Siegel, MD Professor, Sr. Vice Chair, and Chief / Division of Nuclear Medicine / Department of Radiology / Washington University / St. Louis, MO
Jeff M. Michalski, MD Vice Chairman, Radiation Oncology / Chief, Genitourinary Service / Washington University / St. Louis, MO
Clinical history:
Conventional imaging results:
Additional PSA draws:
Planned clinical management:
Details associated with decision to refer patient for an Axumin scan:
Axumin imaging interpretation:
Final management plan:
Clinical follow-up:
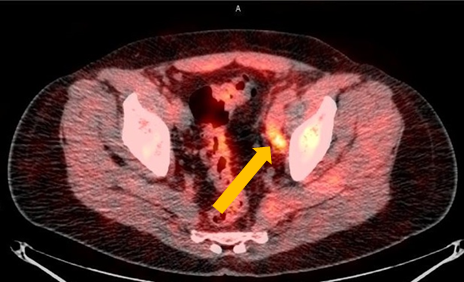
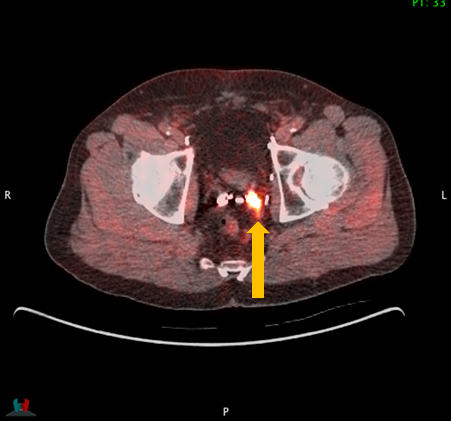
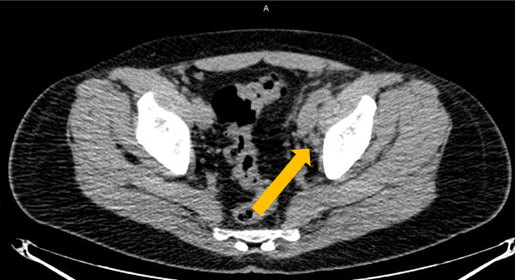
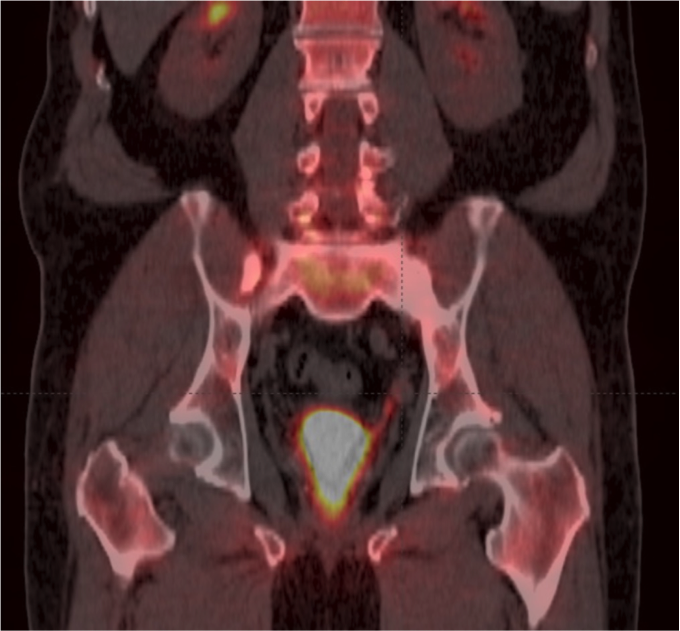
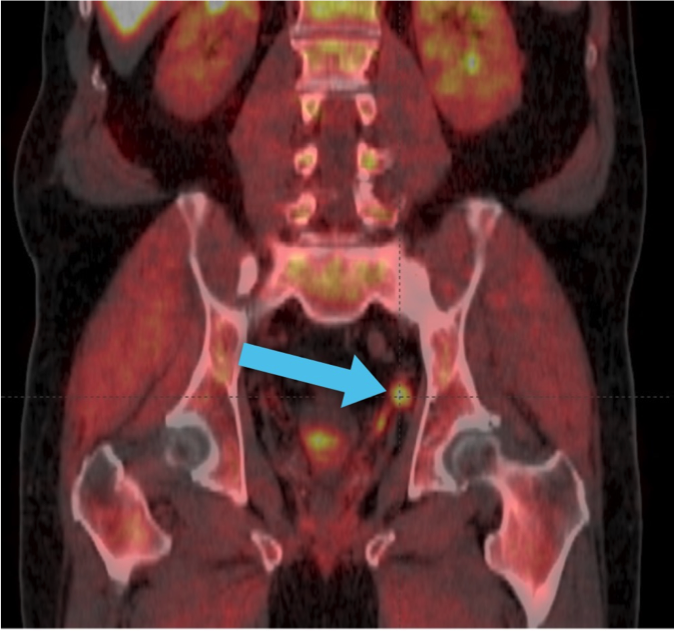
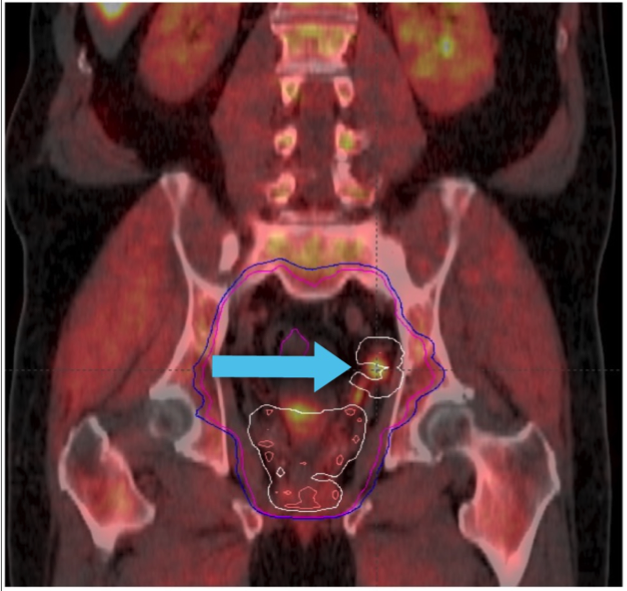
Axumin imaging revealed left obturator lymph node positive uptake (11/2016)
Lymph Node SUV (bw)max: 3.2
Provided by:
Michael Kipper, MD Nuclear Medicine Physician / Genesis Research LLC / San Diego, CA
Paul Dato, MD Urologist / Genesis Research LLC / San Diego, CA
Clinical history:
PSA at recurrence:
Conventional imaging results:
Planned clinical management:
Axumin imaging interpretation:
Final management plan:
Clinical follow-up:
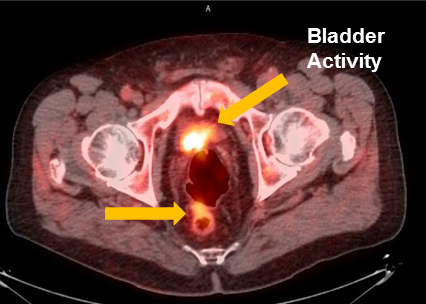
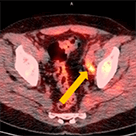
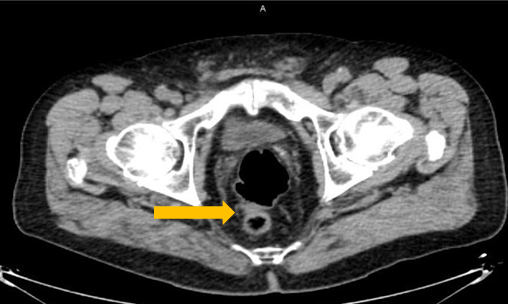
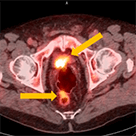
Axumin imaging revealed interior peri-rectal malignant lymph node uptake (6/2016)
Lymph Node SUV(bw)max: 2.8
Blood pool SUV(bw)mean: 2.2
Provided by:
David Josephson, MD, FACS Tower Urology Institute for Minimally Invasive and Robotic Surgery / Attending Surgeon / Cedars Sinai Medical Center / Los Angeles, CA
Jennifer Kujak, MD Director of Oncological Imaging / RadNet Los Angeles / Los Angeles, CA
Clinical history:
PSA at recurrence:
Conventional imaging results:
Planned clinical management:
Details associated with decision to refer patient for an Axumin scan:
Axumin imaging interpretation:
Final management plan:
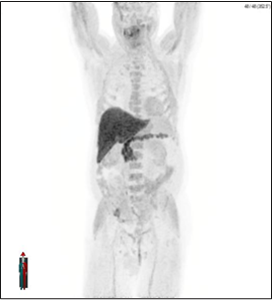
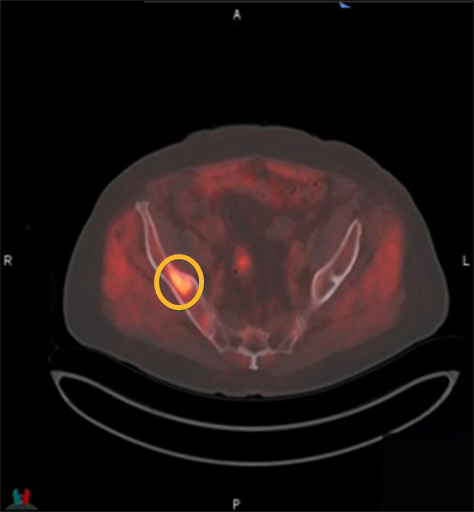
Provided by:
Froylan Gonzalez, MD Board Certified Urologist / Georgia Urology / Canton, GA
Clinical history:
PSA at recurrence:
Conventional imaging results:
Planned clinical management:
Axumin imaging interpretation:
Final management plan:
Clinical follow-up:
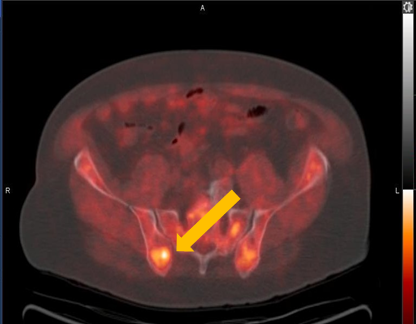
Axumin imaging revealed right Ilium skeletal uptake (8/2016)
Rt. Ilium Lesion SUV(bw)max: 6.0
Marrow (L3) SUV(bw)mean: 3.0
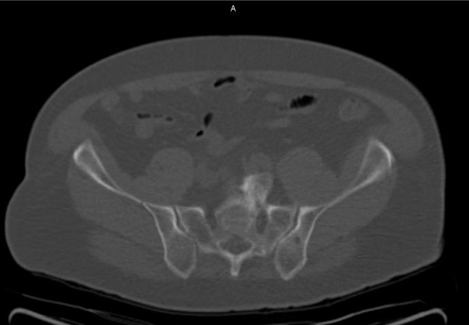
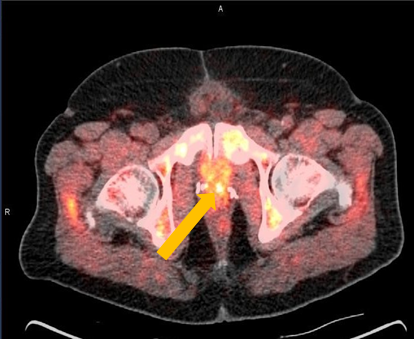
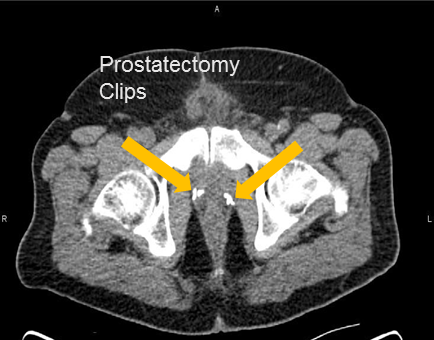
Axumin imaging revealed left prostatectomy bed uptake (8/2016)
Prostatectomy Bed Lesion SUV(bw)max: 4.3
Marrow (L3) SUV(bw)mean: 3.1
Provided by:
Barry A. Siegel, MD Professor, Sr. Vice Chair, and Chief / Division of Nuclear Medicine, Department of Radiology / Washington University / St. Louis, MO
Jeff M. Michalski, MD Vice Chairman, Radiation Oncology / Chief, Genitourinary Service / Washington University / St. Louis, MO
Clinical history:
PSA at recurrence:
Conventional imaging results:
Planned clinical management:
Details associated with decision to refer patients for an Axumin scan:
Axumin imaging interpretation:
Final management plan:
Negative NaF PET/CT Bone Scan
Positive Axumin PET/CT scan
IMRT and daily IGRT to 45.0 Gy/1.8 Gy/25 treatments followed by an additional 23.4 Gy/1.8 Gy/13 treatments to the prostate bed to achieve a total treatment dose of 68.4 Gy/1.8 Gy/38 treatments
Provided by:
Steven Eric Finkelstein, MD, DABR, FACRO Florida Cancer Affiliates / The US Oncology Network
Clinical history:
Additional treatment and PSA draws:
Conventional imaging results:
Planned clinical management:
Details associated with decision to refer patient for an Axumin scan:
Axumin imaging interpretation:
Final management plan:
Clinical follow-up:
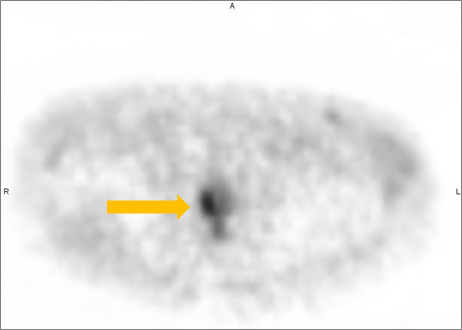
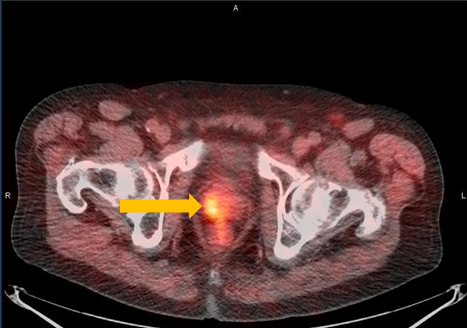
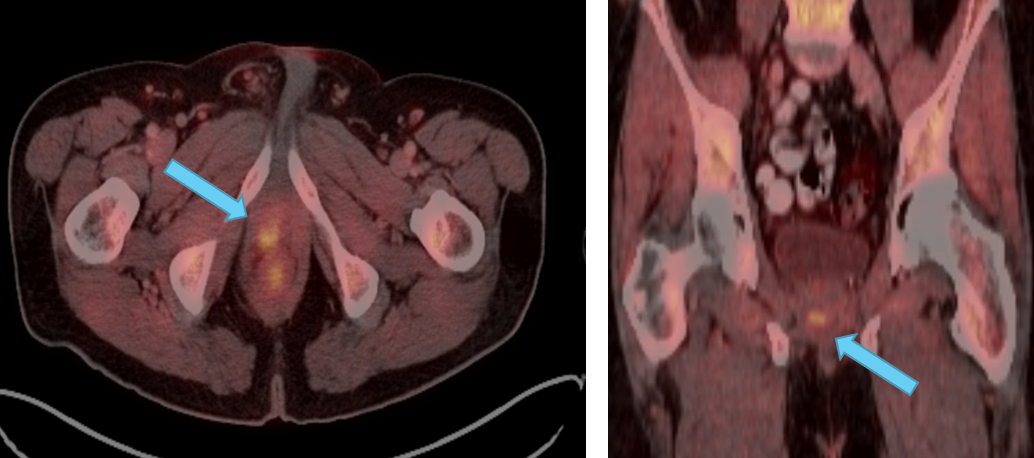
Axumin imaging revealed right mid peripheral zone malignant uptake (7/2016)
Prostate Lesion SUV(bw)max: 3.4
Marrow (L3) Reference SUV(bw)mean: 2.6
Provided by:
Michael Kipper, MD Nuclear Medicine Physician / Genesis Research LLC / San Diego, CA
Paul Dato, MD Urologist / Genesis Research LLC / San Diego, CA
Clinical history:
PSA at recurrence:
Subsequent PSA levels:
Conventional imaging results:
Planned clinical management:
Axumin imaging interpretation:
Final management plan:
Clinical follow-up:
Provided by:
Jonathan Tward, MD, PhD Rudolph P. and Edna S. Reese Endowed Research Professor / Associate Professor of Radiation Oncology / Co-Leader, Genitourinary Malignancies Disease-Oriented Team / Huntsman Cancer Institute at the University of Utah
Clinical history:
PSA at recurrence:
Conventional imaging results:
Planned clinical management:
Details associated with decision to refer patients for an Axumin scan:
Axumin imaging interpretation:
Axumin impact on treatment plan:
Final management plan:
Follow-up and outcomes:
Provided by:
Shawn S. Carter, MD Radiology Regional Center / Fort Myers, Florida
These case studies are being provided to you as examples of the images and information available following Axumin PET/CT imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment. The responsibility for the accurate and timely acquisition and interpretation of images using Axumin PET/CT scanning rests with the nuclear medicine physician or radiologist supervising the PET/CT imaging facility. Axumin is not indicated for directing or changing patient management. These case studies are not intended to substitute for the independent medical judgment of the physician(s) responsible for the individual patient’s management, nor are they a guarantee of any specific clinical results. Incidental findings are noted in some of the cases, as examples of potential, unanticipated abnormalities that may be identified during interpretation of Axumin images. The diagnostic efficacy of Axumin for the identification of these incidental abnormalities has not been established and confirmatory testing may be considered appropriate. These case studies are post marketing, on label, and, at present, there is nothing to suggest that adverse events potentially change Axumin’s safety profile.
Axumin® (fluciclovine F 18) injection is indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment.
To report suspected adverse reactions to Axumin, call 1-855-AXUMIN1 (1-855-298-6461) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Axumin full Prescribing Information.
You are now leaving Axumin.com. Do you wish to proceed?